Content Status
Type
Linked Node
Severity Criteria for Reporting ADRs
Learning ObjectivesSeverity Criteria for Reporting ADRs
H5Content
Content
Adverse Drug Reactions (ADRs) have been graded based on their severity. The figure below provides criteria for the assessment of the severity grade of ADRs.
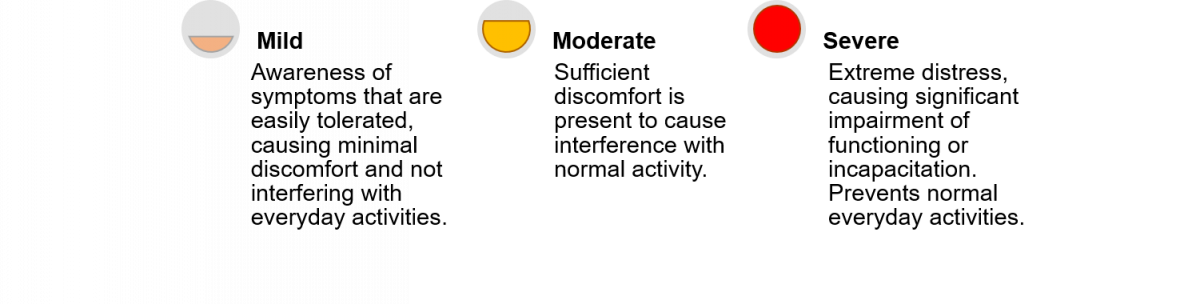
Figure: Criteria for Severity Grade Assessment of ADRs
- The investigator should use their clinical judgment in assessing the severity of events not directly experienced by the subject, e.g., laboratory abnormalities.
- Safety assessment measure is the proportion of patients experiencing a grade 3 or greater adverse event, as defined by Division of AIDS (DAIDS) criteria during treatment and follow-up.
- Please click here for more information on the DAIDS criteria.
Resources
- Guidelines for Programmatic Management of Drug-resistant Tuberculosis in India, March 2021.
- Ready Reckoner for Medical Officer - Adverse Drug Reactions Associated with Anti-TB Drugs Identification and Management, 2019.
- Division of AIDS (DAIDS) Table for Grading the Severity of Adult and Paediatric Adverse Events, 2017.
Kindly provide your valuable feedback on the page to the link provided HERE
LMS Page Link
Content Creator
Reviewer
Target Audience
- Log in to post comments