Content Status
Type
Linked Node
Management of M/XDR-TB Treatment During Pregnancy
Learning Objectives
H5Content
Content
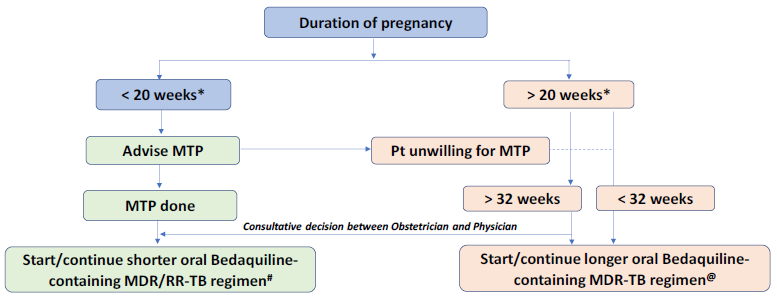
In pregnant women diagnosed with multi-drug resistant (MDR)/rifampicin-resistant TB (RR-TB), if the duration of pregnancy is <20 weeks*, the patient should be advised to opt for medical termination of pregnancy (MTP) in view of the potential severe risk to both the mother and the fetus. Figure 1 shows the management algorithm for pregnant patients based on their gestational age.
|
Figure 1: Management of M/XDR-TB patients during pregnancy based on gestation age; Source: Guidelines for PMDT, India 2021, p.71 |
| * 24 weeks will apply wherever the bill is passed. # Regimen: 4-6 Bdq (6m) Lfx, Cfz, Eto, Hh, Z, E / 5 Lfx, Cfz, Z, E. No modifications allowed. @ Regimen:18-20 Lfx, Bdq(6m or longer) Lzd#, Cfz, Cs. Lzd dose to be tapered to half after 6-8 months based on bacteriological response. Modify regimen if one or more drug cannot be used due to reasons of resistance, tolerability, contraindication, availability etc. • in the order of Z E PAS. • Eto may be considered after 32 weeks’ gestation. • Am may be considered in post-partum period only. Am will not be started in the final 12 months of treatment. |
- If the pregnancy is ≤ 20 weeks* and the patient is willing to opt for MTP, she should be referred to a gynecologist or obstetrician for MTP after which shorter oral Bedaquiline-containing MDR/RR-TB regimen can be initiated (if the patient has not started treatment) or continued (if the patient is already on treatment) by the DR-TB Centre committee.
- If she does not opt for MTP or has a pregnancy >20 weeks* duration, she will be shifted to a longer oral M/XDR-TB regimen.
- For patients who are unwilling for MTP or have a pregnancy of >20 weeks* (making them ineligible for MTP), the risk to the mother and the fetus should be explained clearly and the pregnant DR-TB patients need to be monitored carefully, both in relation to the treatment and progress of the pregnancy.
- Shorter oral Bedaquiline-containing MDR/RR-TB regimen cannot be administered in pregnant women before 32 weeks due to Ethionamide (Eto)-led potential teratogenicity in the first trimester and risk of hypothyroidism in the infant in the second trimester.
- Beyond 32 weeks, the choice of regimen (shorter/longer) needs to be a consultative decision between the obstetrician and physician at the nodal (N)/district DR-TB Centre (DDR-TBC). More compelling evidence on toxicity causes attributed to the use of specific anti-TB drugs during pregnancy and lactation is needed.
Resources
- Guidelines for Programmatic Management of Drug Resistant Tuberculosis in India, March 2021
- Consolidated Guidelines on Tuberculosis: Module 4- Treatment: Drug resistant TB Treatment , 2020
- Technical and Operational Guidelines for TB in India, 2016
- Companion Handbook to the WHO Guidelines for the Programmatic Management of Drug-Resistant Tuberculosis, 2014
Kindly provide your valuable feedback on the page to the link provided HERE
LMS Page Link
Content Creator
Reviewer
Target Audience
- Log in to post comments